INTRODUCTION
Drug development is the process of bringing a new drug molecule into clinical practice.1 Drug development comprises all the activities involved in transforming a compound from a drug candidate (the end-product of the discovery phase) to a product approved for marketing by the appropriate regulatory authorities.2 The drug development phase involves stringent testing and optimization of the selected compounds to identify the ‘drug candidate’ which might be most effective in terms of safety, toxicity, dosage, and efficacy. For this purpose, the selected lead compounds are tested in cells (in vitro) and in animals (in vivo) to study their pharmacodynamic and pharmacokinetic properties, which include Absorption, Distribution, Metabolism, Excretion and Toxicity (ADME/TOX) properties. The successful lead candidate must be non-toxic and should be absorbed into the bloodstream, can be distributed to the proper action site in the body, and can be metabolized efficiently and effectively as well as successfully excreted from the body. This part of the development process is referred to as the ‘Preclinical phase’ in which the drug candidate is meticulously examined, optimized, and prepared for testing in humans. This phase is followed by the ‘clinical phase’ of development, in which the efficacy and safety of a drug candidates is scrutinized in patients. This ‘clinical trial’ has 3 phases:
Phase 1: It perform initial human testing in a small group of healthy volunteers.
Phase 2: It involves testing in a small group of patients.
Phase 3: It includes testing a large group of patients to show safety and efficacy of the drug candidate in them since the healthy and sick people have potentially different metabolic patterns for the drugs.3
Within the different phases of the drug life cycle, drug development is by far the most crucial part for the initial and continued success of a drug on the market.4
Efficacy in drug development is critical for commercial success, for two main reasons:
- Development accounts for about two-thirds of the total R&D costs. The cost per project is very much greater in the development phase, and increases sharply as the project moves into the later phases of clinical development. Keeping these costs under control is a major concern for management. Failure of a compound late in development represents a lot of money wasted.
- Speed in development is an important factor in determining sales revenue, as time spent in development detracts from the period of patent protection once the drug goes to market. As soon as the patents expires, generic competition sharply reduces sales revenue.2
Drug discovery is a complex enterprise bringing together a variety of skills and techniques.5Drug discovery is a lengthy interdisciplinary endeavour.6The process of discovery involves a combination of many disciplines and interests starting from a simple process of identifying an active compound.4 Drug discovery can be described as the process of identifying chemical entities that have the potential to become therapeutic agents. A Key goal of drug discovery campaigns is the recognition of new molecular entities that may be of value in the treatment of diseases that qualify as presenting unmet medical needs.7 Drug discovery has been revolutionized with the advent of genomics, proteomics, bioinformatics, and efficient technologies including combinatorial chemistry, High Throughput Screening (HTS), virtual screening, de novo design, in vitro, in silico ADME screening, and structure-based drug design.6
Drug discovery and development are among the most important translational science activities that contribute to human health and well-being.8 Drug discovery and development is a long-term, competitive, expensive and complicated process. Bringing the drugs from the bench to the market, that is, from screening and identification of the drug to its introduction to the market, takes several of effort. The process of discovering and developing a new drug involves an intricate interaction between investors, industry, academia, patent laws, regulatory authorities, marketing and the necessity to balance confidentiality with communication. The complete process of presenting a drug to the patients involves four stages- drug discovery, drug development, regulatory review and approval, and marketing.3
Drug discovery and development is a knowledge-intensive process that implies the generation, management, and analysis of huge amounts of data from the initial target discovery research to the post-marketing Pharmacovigilence studies, including central operations such as virtual screening and toxicological assessment.9
Pharmaceutical industries have been characterized as firms that discover, develop, manufacture, distribute, and market pharmaceutical products.10 Pharmaceutical industries and regulatory agencies expect that new and better safety biomarkers will play an important role in improving key aspects of the drug development process as well as shortening the time-consuming process and reducing the cost.11
The pharmaceutical industry is highly regulated by the application of the principles of good manufacturing practice (GMP). In most countries, government agencies provide guidance to pharmaceutical manufactures that is intended to facilitate the manufacture of safe, unadulterated and efficacious drug products. The pharmaceutical industry is one of the most highly regulated, and regulation is enforced by governmental and international agencies. 12
The pharmaceutical industry is an important component of healthcare systems throughout the world; it is comprised of many public and private organizations that discover, develop, manufacture and market medicines for human and animal health. The pharmaceutical industry is based primarily upon the scientific research and development (R&D) of medicines that prevent or treat diseases and disorders.
Drug substances exhibit a wide range of pharmacological activity and toxicological properties. Modern scientific and technological advances are accelerating the discovery and development of innovative pharmaceuticals with improved therapeutic activity and reduced side effects. Molecular biologists, medicinal chemists and pharmacists are improving the benefits of drugs through increased potency and specificity. These advances create new concerns for protecting the health and safety of workers within the pharmaceutical industry.
Many dynamic scientific, social and economic factors affect the pharmaceutical industry. Some pharmaceutical companies operate in both national and multinational markets. Therefore, their activities are subject to legislation, regulation and policies relating to drug development and approval, manufacturing and quality control, marketing and sales.
Academic, government and industry scientists, practicing physicians and pharmacists, as well as the public, influence the pharmaceutical industry. Health care providers (e.g., physicians, dentists, nurses, pharmacists, veterinarians) in hospitals, clinics, pharmacies and private practice and private practice may prescribe drugs or recommend how they should be dispensed. Government regulations and health care policies on pharmaceuticals are influenced by the public, advocacy groups and private interests. These complex factors interact to influence the discovery and development, manufacturing, marketing and sales of drugs.
Many countries have specific legal protections for proprietary drugs and manufacturing processes, known as intellectual property rights. In instances when legal protections are limited or do not exist, some companies specialize in manufacturing and marketing generic drugs. The pharmaceutical industry requires large amounts of capital investment due to the high expenses associated with R&D, regulatory approval, manufacturing, quality assurance and control, marketing and sales. Many countries have extensive government regulations affecting the development and approval of drugs for commercial sale. These countries have strict requirements for good manufacturing practices to ensure the integrity of drug manufacturing operations and the quality, safety and efficacy of pharmaceutical products.13
Innovation has always been the backbone and underlying strength of the pharmaceutical industry. During decades the industry has delivered multiple life-saving medicines contributing to new treatment options for several medical needs. Many diseases, particularly acute disorders, are now treatable or can be managed effectively. The discovery of new medications for cardiovascular, metabolic, arthritis, pain, depression, anxiety, oncology, gastrointestinal disorders, women health, infectious diseases and many others have led to improvement in health, quality of life and increased life expectancy.
The decade of 1990s is considered a golden era in the pharmaceutical industry that yielded several blockbusters drugs and lifted the pharmaceutical sector and its select players to top ranks. The years 1996 and 1997 were particularly impressive with record setting approval of 56 and 45 New Molecular Entities (NMEs) and biopharmaceutical entities (NBEs) by USFDA. The large Pharma companies generate the maximal revenues and spend the most in R&D activities. During 2010, the global revenues for pharmaceutical products were 856 billion dollars with US and Europe accounting to approximately 60% of these sales.14
Drug Approval In Unites States
The United States has perhaps the world’s most stringent standards for approving new drugs. Drug approval standards in the United States are considered by many to be the most demanding in the world.15
Drug Approval in Europe
Similar to the US requirements, there are two regulatory steps to go through before a drug is approved to be marked in the European Union. These two steps are clinical trial application and marketing authorization application. There are 27 member states in European Union(as of august 2007); Clinical Trail applications are approved at the member state level, whereas marketing authorization applications are approved at both the member state or centralized levels.16
Drug Approval in Japan
J-NDA submission and review/approval processes and their requirements are similar to those of FDA (Food and Drug Administration) and/or EMA (European Medicines Agency), but there are some important differences that should be noted. For example, there is a mandatory submission of key results of the Japanese population if Japan participates in global studies (or regional studies e.g. Asian studies). This is the most important thing to be understood because substantial programming support is needed. Similar to FDA and EMA, the CSR (clinical study report) and CTD are key documents which need programming involvement for a Japan submission. However, the pooling of adverse events from multiple studies may require inclusion of different indications and thus, may require additional programming support from those who are familiar with PMDA’s requirements.17
DRUG APPROVAL PROCESS IN USA
In the USA, all the food, drugs, cosmetics and medical devices for both humans and animals are regulated under the authority of the United States Food and Drug administration (USFDA). USFDA acts as public health protector in United States and ensures that all drugs in the market are safe and effective.
The Evolution of US Drug Law and Regulations:
United States Pharmacopoeia (USP) was started in 1820 to set standards for strength and purity of drugs. Major milestones in the evolution of US drug law are:
- Food and Drugs Act (1906): It requires that the drugs must meet official standards of strength and purity.
- Federal Food, Drug and Cosmetic Act (1938): It was enacted after sulphanilamide tragedy, to prove the safety of a drug before being marketed.
- Kefauver-Harris Amendment (1962): It was passed after the thalidomide disaster,it requires the manufacturers to prove that drug is safe and effective. All the firms should send adverse effect reports to FDA.
- Orphan drug Act (1973): This allows tax deductions for drug companies to develop orphan drugs.
- Generic drug enforcement Act (1992): It deals with convictions related to ANDA approvals.
- FDA Modernization Act (1997): It contains some changes in Federal Food, Drug and Cosmetic Act regarding collection and assessment of user fees and accelerated approval processes. 18
Investigational New Drug (IND) Application
It’s an application filed to the FDA in order to start clinical trials in humans if the drug was found to be safe from the reports of Preclinical trials. A firm or institution, called a sponsor, is responsible for submitting the IND application.15
Types of IND:
- An Investigator IND: It is submitted by a physician who both initiates and conducts an investigation, and under whose immediate direction the investigational drug is administered or dispensed. A physician might submit a research IND to propose studying an unapproved drug, or an approved product for a new indication or in a new patient population.
- Emergency Use IND: This allows the FDA to authorise use of an experimental drug in an emergency situation that does not allow time for submission of an IND.
- Treatment IND: It is submitted for an experimental drugs showing promise in clinical testing for serious or immediately life-threatening conditions while the final clinical work is conducted and FDA review takes place.18
A pre-IND meeting can be arranged with the FDA to discuss a number of issues:
- The design of animal research, which is required to lend support to the clinical studies.
- The intended protocol for conducting the clinical trial.
- The chemistry, manufacturing, and control of the investigational drug.
Such a meeting will help the sponsor to organize animal research, gather data, and design the clinical protocol based on suggestions by the FDA.16a process of INDA has been illustrated in Figure:1
New Drug Application (NDA):
A New Drug Application (NDA) can be filed only when the drug successfully passes all three phases of clinical trials and comprises all animal and human data, data analyses, pharmacokinetics of drug and its manufacturing and anticipated labeling. The preclinical, clinical reports and risk-benefits analysis are studied at the centre for Drug Evaluation.

If clinical studies confirm that a new drug is comparatively safe and effective, and will not pose irrational risks to patients, the manufacturer files a New Drug Application (NDA), the actual request to manufacture and can market the drug in United States.
Generally, approval of an NDA is granted within two years, however, this process can be finalized from two months to several years. The innovating company is permitted to market the drug after the approval of an NDA and is considered to be in phase IV trails. In this phase, new areas, uses or new populations, long-term effects, and how participants respond to different dosages are explored.19 the process of NDA has been illustrated in the figure: 2

Clinical Trials
Clinical trials are the systematic investigation of the effects of an investigational agent; conducted under stringent conditions and

IND is submitted for one or more clinical phases. Only after the review of an IND by the FDA and a local Institutional Review Board (IRB), human studies can be initiated. An NDA can be filed only when the drug successfully passes the first three phases of clinical trials. The preclinical, clinical reports and risk-benefit analysis are reviewed at the Centre for Drug Evaluation and research (CDER) 18
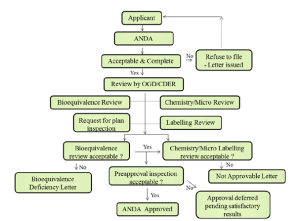
Abbreviated New Drug Application (ANDA)
It’s an application made for approval of generic drugs. The sponsor is required to reproduce the clinical studies that were done for the original, brand name product. Instead, generic drug manufacturers must demonstrate that their product is the same as, and bioequivalent to, a previously approved brand name product.15the process of ANDA has been illustrated in figure:3
Supplemental New Drug Application (SNDA)
After approval of NDA or ANDA, all significant changes in the conditions described in the applications must be approved, by filing a supplemental NDA or ANDA. Such changes like those in packing or ingredients should be approved by CDER. New-uses approvals of already approved drugs coming under this category are better innovation as they need lesser resources to review than that needed for original-use approvals.18
DRUG APPROVAL PROCESS IN EUROPE
In European Union (EU), the medical products were approved for marketing at the National level initially. The mutual reorganization procedure was introduced in 1983 and a single national review in case of pharmaceutical/medicinal product for marketing authorizations in all EU’s countries was made feasible. The initial aim of this procedure was to establish a united standard for product review among national regulatory authorities.
In 1987, for high-technology or biologically derived products, the concentration procedure was established by directive 87/22, in which product assessment should be completed by Committee for Proprietary Medicinal Products (CPMP) besides the normal national regulatory review.
Further, in 1993, by council regulation (EEC) 2309/93, the concentration procedure was replaced with centralized procedure, by which all the high-tech and biologically derived product was reviewed and granted EU’s wide marketing authorization application is filed including all data of animal and human studies, its analyses, as well as pharmacokinetics, manufacturing and proposed labelling.20
Centralized Procedure
The Centralized procedure is one which allows applicants to obtain a marketing authorization that is valid throughout the EU.
- Results in a single authorization valid in EU, Norway, Iceland and Liechtenstein.
- Application evaluated by an assigned Rapporteur.
- Timeline: EMA opinion issued within 210 days, and submitted to European Commission for final approval.
Centralized process is compulsory for:
- Those medicines which are derived from any biotechnology processes, such as genetic engineering.
- Those medicines which are intended for the treatment of cancer, HIV/Aids, diabetes, neurodegenerative disorders or autoimmune diseases and other immune dysfunctions.
- Medicines officially designated ‘orphan medicines’ (medicines used for rare diseases).16
The process of Centralized procedure has been illustrated in figure 4

Decentralized Procedure
In the decentralized procedure, the applicant chooses one country as the reference Member State when making its application for marketing authorization. The chosen reference Member State when making its application for marketing authorization. The chosen reference Member State then prepares a draft assessment report that is submitted to the other member states where approval is sought for their simultaneous consideration and approval.
In allowing the other Member States access to this assessment at an early stage, any issues and concerns can be dealt with quickly without delay, which sometimes is known to occur with the mutual recognition procedure.
Compared with the mutual recognition procedure, the decentralized procedure has the advantage that the marketing authorization in all chosen Member States is received simultaneously, enabling simultaneous marketing of the medicine and reducing the administrative and regulatory burden.
Today, the decentralized procedure is mainly used for applications for generic medicines. As for the mutual recognition procedure, disagreements are handled by CMDh or the CHMP in case no agreement can be reached at CMDh level.21Timeline for this procedure is 210 days.16
The process of Decentralized procedure has been illustrated in Figure 5
National Procedure
Each EU state can have its own procedures for approving drugs that fall outside of those required to undergo the centralized process.22 National procedure is procedure adopted by each nation independently of other nations. The fees are affordable even for small firms. It saves on translation cost to English or regional languages. It creates a base for mutual recognition Procedure Biotechnical procedures cannot be registered through national procedure. The Centralized filing through EMA is compulsory for the same. The application, submitted by the sponsor under the national rules to the national competent authority, is reviewed and a marketing authorization is granted.

Under this scheme also following product cannot be registered: Orphan Medicinal Product, All Biotechnology Based product, Specified Aids and Cancer Medicines, Specified Antiviral Medicines, Specified Medicines for Neurodegenerative Disorder including Diabetes and specified Medicines for Auto Immune Diseases/ dysfunctions.23
Timeline for this procedure is 210 days.16 the national Procedure has been illustrated in figure 6

Mutual Recognition Procedure
The Mutual Recognition Procedure (MRP) is similar to the de-centralized procedure with some differences. The Mutual recognition practice is applicable to medicinal products which have received a marketing authorization in any member state where as the decentralized procedure is applicable to those products which were never approved in any member states of the European Union.
The MRP is used to obtain marketing authorizations in various member states. The assessment of application by RMS can be taken within 90 days instead of 120 days (in decentralized procedure).20
The process may consume a time period of 390 days.16 the Mutual Recognition Procedure has been illustrated in Figure 7.

JAPAN DRUG APPROVAL PROCESS
The MHLW is in charge of pharmaceutical regulatory affairs in Japan (veterinary drugs are under the jurisdiction of the Ministry of Agriculture, Forestry and Fisheries), and the Pharmaceutical Safety and Environmental Health Bureau (PSEHB) undertakes main duties and functions of the Ministry; it handles clinical studies, approval reviews and post-marketing safety measures, i.e., approvals and licensing.
The Health Policy Bureau handles promotion of R&D, production, distribution policies, and drug pricing, i.e., functions related to pharmaceutical companies. The Pharmaceuticals and Medical Devices Evaluation Center (Evaluation Center) in the National Institute of Health Sciences was established to strengthen approval reviews and to introduce a specific system for reviewing tasks for drugs, etc. on July 1, 1997.
To confirm the reliability of reviews and application data, the Organization for Pharmaceutical Safety and Research (OPSR) conducted compliance reviews on application data.24
In accordance with the special corporation rationalization plan passed by the Cabinet in December 2001, and enactment of the Pharmaceuticals and Medical Devices Agency Law in December 2002, the PMDA (KIKO) was established in April 2004, through the integration of the Pharmaceutical and Medical Devices Evaluation Center in the National Institute of Health Sciences, the OPSR, and part of the Medical Devices Center, and the PMDA started handling all consultation and review work from the preclinical stage to approvals and post-marketing surveillance.25
In order to conduct clinical studies to collect data to be submitted with approval applications for new drug manufacturing and marketing, the Act on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics and the GCP require that the MHLW be notified of the study protocol beforehand and provide various requirements to be met by the sponsor when requesting medical institutions to perform clinical studies.
From April 1st, 2011, attachments to the clinical trial notification are required to be submitted in electronic format as well as in
paper format. The range of the GCP covers not only clinical studies on patients, but also Phase I studies in healthy volunteers, bioequivalence studies on humans, studies for additional indications for an approved drug and post-marketing clinical trials after marketing.
At the time of the clinical trial protocol notification, a system by which the PMDA reviews the contents of the initial notification at the request of the MHLW is now specified by law, and a “clinical trial consultation system” in which the PMDA gives guidance and advice concerning study protocols has also been established.26
INVESTIGATIONAL NEW DRUG APPLICATION
In order to protect public health, it is mandatory to submit an IND application to the Ministry of Health, Labor and Welfare. Application documents for a new drug should be prepared in the Common Technical Document (CTD) format following the ICH-M4 guideline.27
The term “sponsor-investigator” as used in the Ministerial Ordinance on Good Clinical Practice for Drugs means an investigator who has submitted a clinical trial notification pursuant to the Act on Securing Quality, Efficacy and Safety of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics, (the Act) in order to conduct a clinical trial at the medical institution etc. to which the investigator belongs (including a coordinating investigator who has submitted a clinical trial notification pursuant to the Act, on behalf of all participating investigators, for a clinical trial conducted according to a single protocol but at more than one medical institution).26

IND APPROVAL PROCESS: 27
The Investigational New Drug Application Approval Process as shown in figure 8

NEW DRUG APPROVAL (NDA) APPLICATION
Procedure
The entire process of approval review from review-related inspections and clinical trial consultation to review works is undertaken by the PMDA (Figure 9, Figure 10). Application forms for drug marketing authorization are submitted to the PMDA.
When application forms for new drugs marketing authorization are received by the PMDA, a compliance review of the application data (certification from source data), GCP on-site inspection, and detailed review are undertaken by review teams of the PMDA and the team prepares a review report.
The approval review process consists of expert meetings of review team members and experts to discuss important problems. A general review conference attended by team members, experts and representatives of the applicant is held after the expert meeting.
It is necessary to submit a “list of persons involved in compilation of attached data” and a “list of competitive products and companies” in relation to persons who participated in clinical studies submitted as application data immediately after application submission, prior to the expert meeting, and prior to meeting of the Committee on Drugs.26

REFERENCES
- Mak, S. S. L. (2019). Mobile Health Technology Use in Vulnerable Populations(Doctoral dissertation, University of California, Los Angeles).
- P.Rang, R.G.Hill, (2013) chapter – 14, Drug development, In Drug discovery and development, second edition; page: 203
- Agrawal, P. (2014). Drug discovery and development: an insight into Pharmacovigilance. Journal of Pharmacovigilance.
- Pratibha Muntha, (2016) Drug discovery and development – A Review, Research and Reviews in Pharmacy and Pharmaceutical sciences.
- Guichard, S. M. (2017). CRISPR–Cas9 for Drug Discovery in Oncology. In Annual Reports in Medicinal Chemistry(Vol. 50, pp. 61-85). Academic Press.
- Rafik karaman, Drug design and Drug discovery, Drug Designing: Open Access, volume 9, Issue 1, page: 1
- Huang, S. M., Lertora, J. J., Markey, S. P., & Atkinson Jr, A. J. (Eds.). (2012). Principles of clinical pharmacology. Academic Press.
- Salazar, D. E., & Gormley, G. (2017). Modern Drug Discovery and Development. In Clinical and Translational Science(pp. 719-743). Academic Press.
- Sanz, F. (2017). Data Integration and Sharing Supporting Drug R&D.
- Osakwe, O., & Rizvi, S. A. (2016). Social aspects of drug discovery, development and commercialization. Academic Press.
- Anadón, A., Castellano, V., & Martínez-Larrañaga, M. R. (2014). Biomarkers in drug safety evaluation. In Biomarkers in Toxicology(pp. 923-945). Academic Press.
- Sandle, T. (2015). Pharmaceutical microbiology: essentials for quality assurance and quality control. Woodhead Publishing.
- Keith D.Tait, Chapter-79 – Pharmaceutical Industry, fourth edition, Encyclopaedia of Occupational Health and Safety
- Khanna, I. (2012). Drug discovery in pharmaceutical industry: productivity challenges and trends. Drug discovery today, 17(19-20), 1088-1102.
- Vishal, P., Rahulgiri, G., Pratik, M., & Kumar, B. J. (2014). A review on drug approval process for US, Europe and India. Int J Drug Regul Affairs, 2(1), 1-11.
- Kashyap, U. N., Gupta, V., & Raghunandan, H. V. (2013). Comparison of drug approval process in United States & Europe. Journal of pharmaceutical Sciences and Research, 5(6), 131.
- Ryan Hara , Novartis Pharma AG, Basel, Switzerland, Japanese Submission/Approval Processes from programming perspective , Pharma SUG 2015 , paper SS02 , page : 2
- K Mahapatra, A., Sameeraja, N. H., & Murthy, P. N. (2014). Drug approval process–in United States of America, European Union and India: a review. Applied Clinical Research, Clinical Trials and Regulatory Affairs, 1(1), 13-22.
- Chakraborty, K., & Yadav, K. (2018). Drug approval process in US, Europe and India and its regulatory requirements: A Review. International Journal of Drug Regulatory Affairs (IJDRA), 6(3), 31-39.
- Shivangi Mukati, (2017) Review Article : Study & Comparison of Drug Approval Process for Different Countries in Relation to Authorization Agency & Clinical Trails, International Journal of Pharmaceutical & Biological Archives, 8(6), page :25-26
- Abed, I. (2014). The approval process of medicines in Europe. Medical Writing, 23, 117-121.
- Van Norman, G. A. (2016). Drugs and devices: comparison of European and US approval processes. JACC: Basic to Translational Science, 1(5), 399-412.
- Adusei-Mensah, F. (2020). Toxicological surveillance and safety profile of commonly used herbal medicinal products in Kumasi metropolis of Ghana(Doctoral dissertation, Itä-Suomen yliopisto).
- Pharmaceutical Administration and Regulations in Japan, Organization and Function of the Ministry of Health, Labour & Welfare, Japan Pharmaceutical Manufacturers Association, 2018, ( JPMA 2018), Chapter 1, page : 1
- Pharmaceutical Administration and Regulations in Japan, Organization and Function of the Ministry of Health, Labour & Welfare, Japan Pharmaceutical Manufacturers Association, 2015, ( JPMA 2015), Chapter 1, page : 3
- Drug Approval System, Drug Approval System of Japan, December 2015, Chapter 4, page : 19,24-25
- Japan Drug Regulatory Overview, 2014, Report, Pacific Bridge Medical 7315 Wisconsin Avenue, suite 609E, Bethesda, MD20814.

