INTRODUCTION
Benzimidazole is a heterocyclic aromatic organic compound. It is very important skeletal in medicinal chemistry. Today this moiety bearing various medicinal characteristics. The most famous benzimidazole derivative in practice is N-ribosyl-dimethylbenzimidazole, which act as ligand bind with Co metal in vitamin B121. The application of Benzimidazole records many year back2. In 1990 different benzimidazole moiety were prepared with different group such as fluorine, tetrahydroquinoline, propylene & ring compoundwhich resulted in drug with bioavailability, increased stability & significant biological potency3,4. In 1991 benzimidazole derivatives were synthesized by derivatization at N-H of benzimidazole by electron donating group and substitution with long chain of propyl, acetamido, thio, thiazole-amino, tetramethyl piperidine on pyridine resulting in good antiulcer activity5,6. Nowadays infectious microbial diseases are causing problems worldwide, because of resistance to number of antimicrobial agents (β-lactam antibiotics, macrolides, quinolones, and vancomycin). A variety of clinically significant species of microorganisms has become an important health problem globally7. One way to fight with this challenge is the appropriate usage of the available marketed antibiotics the other is the development of novel anti-microbial agents8. There is always scope to determine novel chemotherapeutic to remove the emergency need and also release shortage problem of therapy. Due to the structural similarity to purine, antibacterial ability of benzimidazoles are explained by their competition with purines resulting in inhibition of the synthesis of bacterial nucleic acids and proteins9,10.
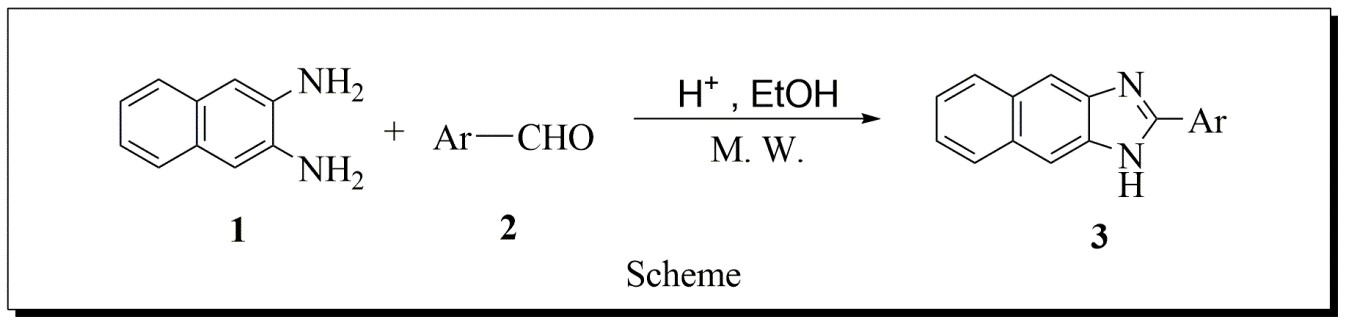
A number of Synthetic strategies have been developed for the preparation of substituted napthaimidazoles. The most common method involve the condensation of an aryl-1,2-diamine with various aldehydes compound in refluxing ethanol for 2–12 h, and this typically gives yields of 34–70 %. Hence, the search for the better method, therefore we have plane reported protocol for one-pot synthesis of naphthaimidazole derivatives from readily available 2, 3-naphthalenediamines and aldehyde under acidic conditions. A wide range of functional groups can be tolerated in the building blocks.
REACTION SCHEME
The cyclocondensation reaction between naphthalene-2,3-diamine (0.0105 mole) and bbenzaldehydes (0.01 mole) in ethanol (10 mL) under Microwave to afford naphthaimidazole A using 5mmol % HCl with respect to naphthalene-2,3-diamine (Scheme 1).

RESULT AND DISCUSSION
Optimization of reaction condition
The cyclocondensation reaction between naphthalene-2,3-diamine (0.0105 mole) 1 and benzaldehyde 2 (0.01 mole) in ethanol (10 mL) under Microwave to afford Napthaimidazoles 3 (Scheme 1) was chosen as the model reaction for optimization. The amount of HCl was used in the ratio of mmole % with respect to naphthalene-2,3-diamine. The reaction was studied under Microwave at power level 240 Watt. Reaction was also optimized by varying HCl (Table 4.1, entries 1-5). It was observed that 5 mmole % amount of catalyst on the basis of naphthalene-2,3-diamine is suitable to complete.
Table 1: Effect of different catalyst on the condensation of naphthalene-2,3-diamine and benzaldehydes in ethanol under microwave.
| Entry | Catalyst | Under Microwave. | |
| Timea
(min) |
Yieldsb
(%) |
||
| 1 | 1mmol % HCl | 2 | 82 |
| 2 | 2mmol % HCl | 2 | 82 |
| 3 | 3mmol % HCl | 2 | 82 |
| 4 | 4mmol % HCl | 3 | 80 |
| 5 | 5mmol % HCl | 3 | 85 |
| 6 | 6 mmol% HCl | 3 | 80 |
a Reaction was monitored by TLC, b Isolated yields.
Our next target of the present work was to synthesize all the napthaimidazole derivatives by taking the assistance of microwave irradiation (MWI) technique using ethanol as the solvent. These series of experiments were also optimized with respect to power levels. The reactions as presented in Scheme 1 were carried out under microwave irradiation. The MWI reactions were carried out in the Scientific Microwave system CATA-R CATALYST SYSTEM. The optimization of the power levels was checked with reference to duration of reaction, yield improvement. The characteristic data are given in Table 2.
Table 2: Data representing the optimization for synthesis of Napthaimidazoles by the assistance of MWI technique.a, b
| Power Levels in Watt | Reaction Time (min)a | % Isolated Yieldb |
| 140 | 4 | 65 |
| 210 | 4 | 80 |
| 240 | 3 | 85 |
| 280 | 3 | 75 |
| 350 | 3.5 | 75 |
aReaction was monitored by TLC.; bIsolated yields.
From the above experimental data, it becomes clear that more efficient results were obtained at 240 W power level of the MW instrument. At this power level Naphthaimidazole derivative were obtained in 85% yield with very good purity in 3 min.
EXPERIMENTAL
Chemicals and Reagents
All chemicals used were of laboratory reagent grade and used without further purification. 2,3-naphthalenediamines, HCl and Aldehydes were obtained from Samir Tech Chem. Pvt. Ltd., Vadodara, India. All the solvents were supplied by Sisco Chem. Pvt. Ltd., Mumbai, India.
Analytical Methods
Determination of M. P. was done by open capillary process and it is uncorrected. 13C NMR & 1H NMR spectra were measure by solutions in DMSO-d6 using Bruker Avance 400 spectrometer having radiofrequency 400 MHz for 1H NMR, & 100 MHz for 13C NMR. Chemical shifts (δ) are expressed in parts per million (ppm) and referenced to the residual protic solvent. FT-IR spectra were recorded on ABB Bomem Inc. FT-IR 3000 spectrophotometer and are expressed in wave numbers (cm-1). The mass spectra (ESI-MS) were recorded on Shimadzu LCMS-2010 spectrometer and Carbon, Hydrogen and Nitrogen were estimated on a PerkinElmer 2400 Series II CHNS/O Elemental Analyzer. All the reactions were monitored by TLC using aluminum sheet precoated with silica gel 60 f254 (Merck).
General Experimental procedure
To a mixture of an 2,3-naphthalenediamine (1 mmol) and benzaldehyde (1 mmol) in ethanol (5mL), 5% w/w HCl with respect to 2,3-naphthalenediamine was added and the mixture was reflux under microwave at optimum power level. The progress of the reaction was monitored by TLC using aluminum sheet precoated with silica gel 60 f254 (Merck). After completion of the reaction, ethyl acetate was added to the solidified mixture. The filtrate was dried over anhydrous Na2SO4. The solvent was evaporated with care and the pure product was obtained. The product obtained had been characterized by FT-IR, 1HNMR, 13CNMR and GC-MS.
(I) Against Staphylococcus aureus:
Maximum activity were found in compounds (C, B &H) zone of inhibition-10.0 m.m. and minimum activity were found in compound (A) zone of inhibition -6.0 m.m
(II) Against Bacillus megaterium:
Maximum activity were found in compounds (C & J) zone of inhibition -12.0 m.m where as minimum activity were found in compound (D) zone of inhibition -5.0 m.m.
Table 3: The characteristic data showing the synthesis of Napthaimidazoles
| Code | Ar | Thermal Conditions | Microwave Irradiation 240 W | ||
| Reaction Timec (h) | Yield (%) | Reaction
Timec (min) |
Yield (%) | ||
| A | C6H5- | 3 | 88 | 3 | 85 |
| B | 4-CH3-C6H4- | 4 | 70 | 3 | 75 |
| C | 4-Cl-C6H4- | 3 | 80 | 3 | 90 |
| D | 2-Cl-C6H4- | 3.5 | 78 | 3 | 88 |
| E | 4-NO2-C6H4- | 3 | 90 | 3 | 90 |
| F | 2-NO2-C6H4- | 3 | 84 | 3 | 89 |
| G | 3-NO2-C6H4- | 3 | 90 | 3 | 90 |
| H | 2-C4H3O- | 3 | 85 | 3 | 85 |
| I | 4-OCH3-C6H4- | 4 | 78 | 4 | 74 |
| J | 2-OH-C6H4- | 3.5 | 78 | 3.5 | 74 |
| K | 3-OH-C6H4- | 3 | 80 | 3.5 | 80 |
| L | 4-OH-C6H4- | 3.5 | 77 | 3.5 | 82 |
| M | 2-COOH-C6H4- | 3.5 | 81 | 3 | 82 |
| N | 4-OH-3-OCH3-C6H3- | 4 | 76 | 3.5 | 75 |
aReaction was monitored by TLC.; bIsolated yields.

(III) Against Escherichia coli:
Maximum activity were found in compounds (C,B & F) zone of inhibition -13.0 m.m and minimum activity were found in compound (D zone of inhibition -3.0 m.m
(IV) Against Proteus vulgaris
Maximum activity were found in compound (C) zone of inhibition -15.0 m.m (near to standard drug) and minimum activity were found in compounds (D) zone of inhibition 4.0 m.m.
CHARACTERIZATION
2-phenyl-1H-naphtho[2,3-d]imidazole A
IR (KBr): 3433 (w), 3050, 1446, 1412, 1280, 970, 750 cm-1; 1H NMR (400 MHz, DMSO, δ ppm): 12.94 (s, 1H, NH), 8.21 (d, 2H, J=7.6 HZ, Ar-H), 7.62-7.21 (m, 9H, Ar-H); 13C NMR (DMSO-d6, δ ppm): 151.7, 140.1, 130.6, 130.2, 129.4, 126.9, 122.5, 116.4; ESI-MS: m/z (M+H)+ 312.10; Anal. Calcd. for C17H12N2: C, 83.58; H, 4.95; N, 11.47 ; Found: C, 83.52; H, 4.96; N, 11.53.
CONCLUSION
We have reported green protocol for one-pot synthesis of napthaimidazoles from readily available 2,3-naphthalenediamines and various aromatic aldehydes by a simple and convenient protocol. The conditions are mild and a wide range of functional groups can be tolerated in the building blocks for synthesized products. Antimicrobial activity of all the synthesized compounds was done and compare with standards. Compounds C, B, H and F shows good activity.
REFERENCES
- Barker, H. A., Smyth, R. D., Weissbach, H., Toohey, J. I., Ladd, J. N., & Volcani, B. E. (1960). Isolation and properties of crystalline cobamide coenzymes containing benzimidazole or 5, 6-dimethylbenzimidazole. Journal of Biological Chemistry, 235(2), 480-488.
- Patil, A., Ganguly, S., & Surana, S. (2008). A systematic review of benzimidazole derivatives as an antiulcer agent. Rasayan J Chem, 1(3), 447-460
- KUBo, K., ODA, K., KANEKO, T., SATOH, H., & NOHARA, A. (1990). Synthesis of 2-[[(4-fluoroalkoxy-2-pyridyl) methyl] sulfinyl]-1H-benzimidazoles as antiulcer agents. Chemical and pharmaceutical bulletin, 38(10), 2853-2858.
- Uchida, M., CHIHIRO, M., MORITA, S., YAMASHITA, H., YAMASAKI, K., KANBE, T., & NAKAGAWA, K. (1990). Synthesis and Antiulcer Activity of 4-Substituted 8-[(2-Benzimidazolyl) sulfinylmethyl]-1, 2, 3, 4-tetra-hydroquinolines and Related Compounds. Chemical and Pharmaceutical Bulletin, 38(6), 1575-1586.
- Grassi, A., Ippen, J., Bruno, M., & Thomas, G. (1991). BAY P 1455, a thiazolylaminobenzimidazole derivative with gastroprotective properties in the rat. European journal of pharmacology, 195(2), 251-259.
- Özkay, Y., Tunalı, Y., Karaca, H., & Işıkdağ, İ. (2010). Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. European journal of medicinal chemistry, 45(8), 3293-3298.
- He, Y., Wu, B., Yang, J., Robinson, D., Risen, L., Ranken, R., & Swayze, E. E. (2003). 2-Piperidin-4-yl-benzimidazoles with broad spectrum antibacterial activities. Bioorganic & medicinal chemistry letters, 13(19), 3253-3256.
- Metwally, K. A., Abdel-Aziz, L. M., Lashine, E. S. M., Husseiny, M. I., & Badawy, R. H. (2006). Hydrazones of 2-aryl-quinoline-4-carboxylic acid hydrazides: Synthesis and preliminary evaluation as antimicrobial agents. Bioorganic & medicinal chemistry, 14(24), 8675-8682.
- Spasov, A. A., Yozhitsa, I. N., Bugaeva, L. I., & Anisimova, V. A. (1999). Benzimidazole derivatives: Spectrum of pharmacological activity and toxicological properties (a review). Pharmaceutical Chemistry Journal, 33(5), 232-243.
- Arjmand, F., Mohani, B., & Ahmad, S. (2005). Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu (II) complex. European journal of medicinal chemistry, 40(11), 1103-1110.