INTRODUCTION
Several groups of pharmaceutical scientists and technologists from academic or research institution and pharmaceutical industry have been working with different polymers with different drug or active pharmaceutical ingredients (API) for designing and development of formulations.
The pharmaceutical research laboratory and industry have constantly been engaged in devising dosage forms based on polymers specially mucoadhesive polymers with the objectives of prolongation of residence period of formulations. Polymeric materials play an important role in bioadhesion of dosage forms as well as controlling drug release from dosage forms for maintaining the effective or therapeutic blood levels of drugs in the patient’s system for prolong period without causing dose dumping. The study deals with the mucoadhesive polymers and dosage forms design and development based on the mechanism of adhesion1 between polymers used in formulations and mucosal surface. The main goal of this mechanism is to adhere and place the dosage form in a particular site of absorption or site of action for achieving prolong action. Mucosal drug delivery systems through buccal, sublingual, oral, nasal, rectal, vaginal, ocular delivery made of mucoadhesive polymers play an important role by special placement for systemic or local action by interaction with the mucus layer of the site of application because mucosa has a rich blood supply and it is relatively permeable2. Mucoadhesive polymers used in formulation should be able to release the content drug at a constant rate over a long period of time and should be interacting, compatible, non toxic, non irritable with biological system (mucus). Mucoadhesive dosage forms play an important role in enhancement of bioavailability as well as therapeutic efficacy of the content drug or drugs through controlled release at a predetermined rate. Mucoadhesive polymers specially obtained from edible plants or vegetables have been utilized in designing formulations in such a way that make the drug available for the target, providing the sufficient release rate and prolong duration to produce the desired effect. Mucoadhesive formulations is now very much important and glorious fields among the several controlled drug delivery strategies such as matrices, pellets, floating systems, liposomes, microemulsions, liquid crystals, solid dispersions, nanoparticles, nanosuspensions, transdermal systems, cyclodextrin inclusion complexes, osmotic pumps, etc. Mucoadhesive drug delivery system (MDDS) is based on the property of bioadhesion of certain polymers which shows adhesive properties on hydration; therefore helps in targeting a drug to a particular site for desirable period of time. The goal of any drug delivery system is to provide a therapeutic or effective amount of the drug to the proper site in the body to promptly achieve drugs action and then maintain the desired drug concentration for long time with minimization of untoward actions. The special placement of the drug delivery system can satisfy the same aspect. The development of sustain release dosage form can achieve the aim of releasing the drug slowly for a long period but this is not sufficient to get sustained therapeutic effect3. They may be cleared from the site of absorption before emptying the drug content. Instead, the mucoadhesive dosage form will serve both the purposes of sustain release and presence of dosage form at the site of absorption. In this regard, we have been performing some mucoadhesive approaches for developing dosage forms is high lighting few aspects of mucoadhesive drug delivery systems. In the recent years the interest is growing to develop a drug delivery system with the use of a mucoadhesive polymer that will attach to related tissue or to the surface coating of the tissue for the targeting various absorptive mucosa such as buccal, sublingual, gastro-intestinal, ocular, nasal, pulmonary and vaginal3.
Mucus Layer of Different Body Parts
Mucus is translucent and viscid secretion which forms a thin, continuous gel blanket adherent to the mucosal epithelial surface4. The mean thickness of this layer varies about 50 to 450 mm in humans (figure 1). It is secreted by the goblet cell lining the epithelia or by special exocrine glands with mucus cells acini. This tissue layer responsible for formation of the adhesive interface is mucus.

Characteristics and Composition of Mucus
The composition of mucus varies widely depending on animal species, anatomical location, and whether the tissue is in a normal or pathological state. Native mucin, in addition to mucus, also contains water, electrolytes, bacteria, bacterial byproducts and other debris. The glycoprotein fraction of the mucus imparts a viscous or gel like characteristics to mucus due to its water retention capacity, i.e., it holds water 40 times of its weight.
Mucus composition:
Water 95%
Glycoproteins and lipids 0.5 – 5%
Mineral salts 1%
Free proteins 0.5 – 1%
Mucus is a glycoprotein, chemically consisting of a large peptide backbone with pendant oligosaccharide side chains whose terminal end is either sialic or sulphonic acid or L-fucose. The oligosaccharide chains are covalently lined to the hydroxy amino acids, serine and threonine, along the polypeptide backbone5.
About 25% of the polypeptide backbone is without sugars, the so called ‘naked’ protein region, which is especially prone to enzymatic cleavage. The region being rich in charged amino acids, chiefly aspartic acid, is involved in cross…linking via disulphide bonds between mucin molecules. The remaining 75% of the backbone is heavily glycosylated. A highly extended and flexible molecular conformation is suggested for mucus glycoproteins to permit maximum ability to sorb water. The terminal sialic groups have pKa values of 2.6, so that the mucin molecule should be viewed as a poly electrolyte under neutral or slightly acidic conditions. At physiological pH the mucin network may carry a significant negative charge because of the presence of sialic acid and sulphate residues and this high charge density plays an important role in mucoadhesion.
Mucus glycoproteins are high molecular proteins possessing attached oligosaccharide unit. These units contain an average of about 8 – 10 monosaccharide residues of five different types. They are (a) L – fucose, (b) D – galactose, (c) N-acetyl-D-glucosamine, (d) N – acetyl-D-galactosamine, and (e) sialic acid. In humans the
only important sialic acid is N – acetylneuramic acid, although in animals a number of sialic acids occur, including N-glycollyneuramic acid and various O-substituted derivatives. Amino acids are principally serine, threonine, and proline. The mucus layer which covers the epithelial surface has various roles.
Various Roles of Mucus Layer of Different Body Parts4
Protective Role
The protective role results particularly from its hydrophobicity and protecting the mucosa from the lumen diffusion of hydrochloric acid from the lumen to the epithelial surface.
Barrier Role
The mucus constitutes a diffusion barrier for molecules, and especially against drug absorption. Diffusion through the mucus layer depends largely on the physico-chemical characteristics of the active ingredient such as molecule charge, hydration radius, ability to form hydrogen bonds, and molecular weight. The concentration and volume of glycoprotein affects the diffusion rate of drugs. A large number of active ingredients may interact with the mucus, particularly antibiotics. It seems that the formation of insoluble complexes would occur impeding resorption by the gastrointestinal tract as well as by the sub-maxillary route. Gastric mucus may act as an unstirred water layer, in which hydrogen ions diffusing from the lumen are neutralized by the bicarbonate of the surface epithelium secretion. A dynamic equilibrium exists at the mucosal surface between continuous erosion by proteolysis and mechanical abrasion and the equally continuous new mucus secretion.
Adhesion Role
Mucus has strong cohesional properties and firmly binds to the epithelial cell surface as a continuous gel layer and the gel obviously behaves as a non-Newtonian fluid. Mucus layer is responsible for retaining the drug formulations on formation of the adhesive bonding to the drug products based on adhesive materials.
Lubrication Role
The mucus layer keeps the mucosal membrane moist. Continuous secretion of mucus from the goblet cells is necessary to compensate for the removal of the mucus layer due to digestion, bacterial degradation, and solubilization of mucin molecules. At physiological pH the mucus network may carry a significant negative charge because of the presence of sialic acid and sulphate residues and this high charge density due to negative charge contributes significantly to bioadhesion.
Adhesion, Bioadhesion, Mucoadhesion and Mucoadhesive Drug Delivery Systems
Adhesion can be defined as the bond produced by contact between a pressure sensitive material and a surface6. Adhesion is also defined as the state in which two surfaces are held together by interfacial forces which may consist of valence forces, interlocking action or both7.
Bioadhesion is an interfacial phenomenon in which two materials, at least one of which is biological in nature, are held together for an extended period of time by means of interfacial forces8,9. The attachment could be between an artificial material (synthetic or natural) and biological substrate, such as the adhesion between polymer and /or copolymer and a biological membrane. In biological systems four types of bioadhesion can be distinguished.
- Adhesion of a normal cell on another normal cell,
- Adhesion of a cell with a foreign substance,
- Adhesion of a normal cell to a pathological cell,
- Adhesion of an adhesive to a biological substrate.
In the case of adhesion of bioadhesive agents or polymer attached to the mucin layer of mucosal tissues, the term “mucoadhesion” is employed. Mucoadhesion in drug delivery systems has recently gained interest among pharmaceutical scientists as a mean of promoting the residence
time as well as improving intimacy of contact of dosage form with various absorptive membranes of the biological systems10. Besides acting as platforms for sustained – release dosage forms, bioadhesive agents or polymers can themselves exert some control over the rate and amount of drug release and thus contribute to the effective or therapeutic advantage of such systems.
Bioadhesives and Mucoadhesives
A bioadhesive is defined as substance that is capable of interacting with biological materials and being retained on them or holding them together for extended periods of time. Bioadhesives are classified into three types based on phenomenological observation, rather than on the mechanisms of bioadhesion.
- Bioadhesion is characterized by adhesion occurring between biological objects without involvement of artificial materials. Cell fusion and cell aggregation are good examples.
- Bioadhesion can be represented by cell adhesion onto culture dishes or adhesion to a variety of substances including metals, woods and other synthetic materials.
- Bioadhesion can be described as adhesion of artificial substances to biological substrates such as adhesion of polymers or mucoadhesive agents to skin or other soft tissues. The goal of the development of bioadhesive is to duplicate, mimic or improve biological adhesives. They should be both durable where required and degradable where necessary and not toxic at all.
Mucoadhesives are synthetic or natural polymers or adhesive agents which interact with the mucus layer covering the mucosal epithelial surface and mucin molecules constituting a major part of mucus. The concept of mucoadhesives has alerted many investigators to be possibility that these adhesive agents or polymers can be used to overcome physiological barriers in long term drug delivery. They render the treatment more effective and safe, not only for topical disorders but also for systemic problems.
Mechanism of Mucoadhesion
At physiological pH mucin network of mucus layer plays an important role in mucoadhesion10. Mucoadhesion occur by the following phenomena. The first stage involves an intimate contact between a bioadhesive and a membrane, either from a good wetting of the bioadhesive surface or from the swelling of the bioadhesive. In the second stage, after contact is established, penetration of the bioadhesive into the crevice of the tissue surface or interpenetration of the chains of the bioadhesive with those of the mucus takes place. Low Chemical bonds can then settle.
One of the most important factors for bioadhesion9 is tissue surface roughness. Castellanos et al. showed that adhesive joints may fail at relatively low applied stresses if cracks, air bubbles, voids, inclusions, or other surface defects are present11. Viscosity and wetting power are the most important factors for satisfactory bioadhesion. Wachem et al. studied in vitro interaction of human endothelial cells with polymeric or adhesive substances processing different metabolites in a culture medium contain serum12.
On a molecular level, mucoadhesion can be explained on the basis of molecular interactions. The interaction between two molecules is composed of attraction and repulsion. Attractive interactions arise from Vander Waals forces, electrostatic attraction, hydrogen bonding, and by hydrophobic interaction. Repulsive interactions occur because of electrostatic and steric repulsion. For mucoadhesion to occur, the attractive interaction should be larger than nonspecific repulsion.
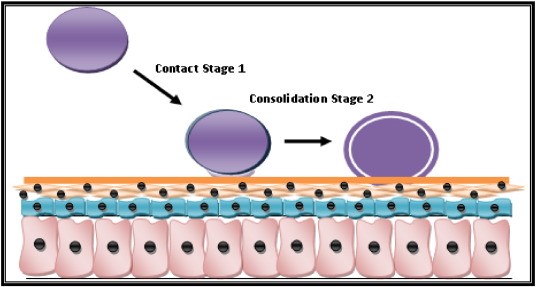
Theories of Bioadhesion and Mucoadhesion
Several theories have been proposed to explain the fundamental mechanisms of adhesion. In a particular system, one or more theories can equally well explain or contribute to the formation of bioadhesive bonds.
Adsorption Theory
According to the adsorption theory, after an initial contact between two surfaces, the material adheres because of surface forces acting between the atoms in the two surfaces13. Two types of chemical bonds resulting from these forces can be distinguished.
- Primary chemical bonds of covalent nature, which are undesirable in bioadhesion because their high strength may result impermanent bonds.
- Secondary chemical bonds having many different forces of attraction, including electrostatic forces, Vander Waals forces, and hydrophobic bonds.
Wetting Theory
Wetting theory is predominantly applicable to liquid bioadhesive systems. It analyses adhesive and contact behaviour in terms of the ability of a liquid or paste to spread over a biological system14.


The work of adhesion (expressed in terms of surface and interfacial tension, Y) is defined as the energy per square centimeter released when an interface is formed. The work of adhesion is given by
Wa = YA + YB – YAB
Where A and B refer to biological membrane and the bioadhesive formulation respectively. The work of cohesion is given by
WC = 2 YA or YB.
For a bioadhesive material B spreading on a biological substrate A, the spreading coefficient is given by
SB/A = YA – (YB + YAB)
SB/A should be positive for a bioadhesive material to adhere to a biological membrane.
Electronic Theory
According to the electronic theory, electron transfer occurs upon contact of an adhesive agents or polymer with a mucus glycoprotein network because of differences in their electronic structures. This results in the formation of an electrical double layer at the interface. Adhesion occurs due to attractive forces across the double layer14.
Diffusion Theory
According to diffusion theory, the adhesive agents or polymer chains and the mucus mix to a sufficient depth to create a semi- permanent adhesive bond. The exact depth to which the adhesive agents or polymer chains penetrate the mucus depends on the diffusion coefficient and the time of contact15.

This diffusion co-efficient, in turn, depends on the value of molecular weight between cross-links and decreases significantly as the cross linking density increases.
Fracture Theory
Fracture theory attempts to relate the difficulty of separation of two surfaces after adhesion. Fracture theory equivalent to adhesive strength is given by G = (E x/L)1/2
Where E is the Young’s modulus of elasticity, x is the fracture energy, and L is the critical crack length when two surfaces are separated15.

Factors Important to Mucoadhesion and Mucoadhesive Drug Delivery Systems
The bioadhesive power of a polymer or mucoadhesive agents or of a series of polymers or mucoadhesive agents is affected by the nature of the polymer or mucoadhesive agent and also by the nature of the surrounding media16,17.
- Polymer or Mucoadhesive Agents – Related Factors
Molecular Weight
Numerous studies have indicated that there is a certain molecular weight at which bioadhesion is at a maximum. The interpenetration of polymer or adhesive molecules is favourable of low molecular weight polymers or mucoadhesives, whereas entanglements are favoured for high molecular weight polymers. The optimum molecular weight for the maximum bioadhesion depends on the type of polymer or adhesive agent. Their nature dictates the degree of swelling in water, which in turn determines interpenetration of polymer or adhesive molecules within the mucus. According to Gurney et al, it seems that the bioadhesive agent or force increases with the molecular weight of the bioadhesive polymer up to 1,00,000 and that beyond this level there is not much effect. To allow chain interpenetration, the polymer or adhesive molecules are also important factors. For example with polyethylene oxide adhesive strength increases even up to molecular weights of 40,00,000; these polymers are well known to contain molecules of highly linear configuration, which contribute to interpenetration with dextran. Molecules with molecular weights as high as 1,95,00,000 do not exhibit better bioadhesion than molecules with a molecular weight of 2,00,000.
Concentration of Active Polymer or Mucoadhesive Agent
Bremecker related that there is an optimum concentration of polymer or mucoadhesive agent corresponding to the best bioadhesion. In highly concentrated systems, the adhesive strength drops significantly. In fact, in concentrated solutions, the called molecules become solvent poor and the chains available for interpenetration are not numerous. This result seems to be of interest only for more or less liquid bioadhesive forms. Duchene et al. for solid dosage forms such as tablets, showed that the higher the polymer or mucoadhesive agent concentration, stronger the bioadhesion.
Flexibility of Polymer or Mucoadhesive Chains
Flexibility is important for interpenetration and entanglement. A water-soluble polymer or Mucoadhesive agent become cross – linked the mobility of the individual polymer or mucoadhesive chain decreases. As the cross…linking density increases, the effective length of the chain which can penetrate into the mucus layer decreases even further and mucoadhesive strength is reduced.
Spatial Conformation
Besides molecular weight or chain length, spatial conformation of a molecule is also important. Despite a high molecular weight of 1,95,00,000 for dextrans, they have adhesive strength similar to that of polyethylene glycol, with a molecular weight of 2,00,000. The helical conformation of dextran may shield many adhesively active groups, primarily responsible for adhesion, unlike PEG polymers, which have a linear conformation.
- Environment or Surrounding Media – Related Factors
pH
pH was found to have a significant effect on mucoadhesion as observed in studies of poly-acrylic polymers cross- linked with –COOH groups. PH influences the charge on the surface of both mucus and the polymers. Mucus will have different charge density depending on pH because of differences in dissociation of functional groups on the carbohydrate moiety and amino acids of the polypeptide backbone.
Robinson and his group observed that the pH of the medium was critical for the degree of hydration of highly cross – linked polyacrylic acid polymers, increasing between pH 4 and pH 5, continuing to increase slightly at pH 6 and pH 7 decreasing at more alkaline pH levels. This behaviour was attributed to differences in charge density at the different pH levels18.
Polycarbophil shows maximum adhesive strength at pH 3; the adhesive strength decreases gradually as the pH increases up to pH 5. Polycarbophil does not show any mucoadhesive property above pH 5. This study the first systematic investigation of the mechanism of mucoadhesion, clearly shows that protonated carboxyl groups rather than ionized carboxyl groups react with mucin molecules, presumably by numerous simultaneous hydrogen bonding reactions19. At pH above 5, poly carbophil swells to larger extent than at pH 3 or below. At high pH, however the chains are fully extended because of the electrostatic repulsion of carboxylate anions. The polymer mucoadhesive chains are also repelled by the negative charged mucin molecules. It has also been observed that, due to hydrogen bonding between hydroxypropyl cellulose and carbopol 934, interpolymer complexes form at pH values below 4.5.
Applied Strength
To place a solid bioadhesive system, it is necessary to apply a defined strength. Whatever the polymer, poly [acrylic acid / divinyl benzene poly (HEMA)] or Carbopol 934, the adhesion strength increases with the applied strength or with the duration of its application, up to an optimum. The pressure initially applied to the mucoadhesive tissue contact site can affect the depth of interpenetration. If high pressure is applied for a sufficiently long period of time, polymers become mucoadhesive even though they do not have attractive interactions with mucin.
Initial Contact Time
The initial contact time between mucoadhesives and the mucus layer determines the extent of swelling and the interpenetration of polymer chains. Along with the initial pressure, the initial contact time can dramatically effect the performance of a system. The mucoadhesive strength increases as the initial contact time increases. However, longer initial contact time should be based on tissue viability. In case of mucoadhesives that need to be polymerized at the application sites, the initial contact time is critical. It is easily controlled when mucoadhesives are applied to exposed areas such as eye, nose or mouth. For the application of mucoadhesives to the gastrointestinal tract, however, the initial contact time cannot be controlled, which is one of the difficulties in applying mucoadhesive to the Gastrointestinal tract20.
Selection of the Model Substrate Surface
The handling and treatment of biological substrates during the testing of mucoadhesives is an important factor, since physical and biological changes may occur in the mucus gels or tissues under the experimental conditions. The viability of the biological substrate should be confirmed by examining properties such as permeability, electrophysiology, or histology. Such studies may be necessary before and after performing the in vitro tests using tissues.
Swelling
The swelling characteristic is related to the polymer itself, and also to its environment. Interpenetration of chains is easier as polymer chains are disentangled and free of interaction. Swelling depends both on polymer concentration and on water presence. When swelling is too great, a decrease in bioadhesion occurs; such a phenomenon must not occur too early, in order to lead to a sufficient action of the bioadhesive system. Its appearance allows easy detachment of the bioadhesive system after the discharge of the active ingredient.
- Physiological Variables
Mucin Turnover
The natural turnover of mucin molecules from the mucus layer is important for at least two reasons. First, the mucin turnover is expected to limit the residence time of the mucoadhsive on the mucus layer. No matter how high the mucoadhesive strength, mucoadhesives are detached from the surface due to mucin turnover. The turnover rate may be different in the presence of mucoadhesives, but no information is available on this aspect. Second, mucin turn over results in substantial amounts of soluble mucin molecules. These molecules interact with mucoadhesives before they have a chance to interact with mucus layer. Surface fouling is unfavourable for mucoadhesion to the tissue surface. Mucin turnover may depend on other factors such as the presence of food. The gastric mucosa accumulates secreted mucin on the luminal surface of the tissue during the early stages of fasting. The accumulated mucin is subsequently released by freshly secreted acid as simply by the passage of ingested food; the exact turnover rate of the mucus layer remains to be determined. The calculated mucin turnover10 time is 47 – 270 min. The ciliated cells in the nasal cavity are known to transport the mucus to the throat at a rate of 5 mm/ min. The mucociliary clearance in the tracheal region has been found to be in the range of 4 –10 mm / min.
Diseases State
The physiochemical properties of the mucus are known to change during disease conditions such as the common cold, gastric ulcers, ulcerative colitis, cystic fibrosis, bacterial and fungal infections of the female reproductive, and inflammatory conditions of the eye. The exact structural changes taking place in mucus under these conditions are not clearly understood. If mucoadhesives are to be used in the disease states, the mucoadhesive property needs to be evaluated under the same conditions.
Use of Mucoadhesive Preparations
The idea of mucoadhesives was derived from the need to localize drugs at a certain site in the body. Often the extent of drug absorption is limited by the residence time of drug at the absorption site. For example, in ocular drug delivery, less than 2 minutes are available for drug absorption after instillation of a drug solution into the eye, since it is removed rapidly by solution drainage, hence the ability to extend contact time of an ocular drug delivery system in front of the eye would undoubtedly improve drug bioavailability. In oral or gastrointestinal drug delivery, the drug absorption is limited by the gastrointestinal transit time of the dosage form20. Since many drugs are absorbed only from the upper small intestine, localizing oral drug delivery system in the stomach or in the duodenum would significantly improve the extent of drug absorption.
Since most of the routes of drug administration, such as ocular, nasal, buccal, respiratory, gastrointestinal, rectal and vaginal routes, are coated with the mucus layer, mucoadhesives are expected increase the residence time. In addition, they provide intimate contact between a dosage form and the absorbing tissue, which may result in high drug concentration in a local area and hence high drug flux through the absorbing tissue. Furthermore, the intimate contact may increase the total permeability of high molecular weight drugs such as peptides and proteins.
Controlled Release Mucoadhesive Drug Delivery Systems
The pharmaceutical scientists and technologists have constantly been engaged in devising dosage forms with the objective of maintaining the effective or therapeutic blood levels of drugs in the patient’s system for prolonged periods without causing dose dumping. Substantial effort has recently been focused on placing a drug or drug delivery system in a particular region of the body for extended period of time with the help of some natural mucoadhesive agents obtained from edible plant sources. This need is not only for local targeting of drugs but also to better control systemic drug delivery21.
In this study a number of natural mucoadhesive agents were collected from edible plants by extraction screened and evaluated by comparison with other established marketed polymers for design and development of controlled release mucoadhesive drug delivery systems1.
Controlled release mucoadhesive drug delivery systems utilize the property of bioadhesion of certain water soluble polymers or mucoadhesive agents which become adhesive on hydration and hence can be used for targeting a drug to a particular region of the body for extended period of time. The mucosal layer lies a number of regions of the body including the gastrointestinal tract, the urogenital tract, the airways, the ear, nose and eye. These represent potential sites for attachment of any bioadhesive systems and hence, the mucoadhesive drug delivery systems may include the following –
- Buccal delivery systems
- Sublingual delivery systems
- Vaginal delivery systems
- Rectal delivery systems
- Nasal delivery systems
- Ocular delivery systems
- Oral Mucoadhesive Drug delivery systems
Advantages of Mucoadhesive Drug Delivery Systems
Mucoadhesive drug delivery system satisfies several features of controlled release systems: –
- It localizes drug in a particular regions thereby improving and enhancing bioavailability; for those drugs with bioavailability problems.
- The strong interaction between the polymer or mucoadhesive agents and the mucus lining of the tissue helps to increase contact time and permit localization, an essential issue when modification of tissue permeability is important for delivery e.g. peptides/proteins and ionized species.
- To inhibit metabolizing enzymes in a localized area.
- To delivery agents locally for the purpose of modulating antigenicity4.
Control Release Oral Mucoadhesive Drug Delivery Systems
A primary objective of using mucoadhesive formulations orally would be to achieve a substantial increase in residence of the drug in the gastrointestinal tract. In 1985, Longer et al. have shown that albumin beads containing chlorthiazide when mixed with equal sized particles of polycarbophil, and administered orally in the form of capsules to rats, showed a longer duration of action and greater bioavailability in comparison to control beads or drug alone18. The mucoadhesive polymer in the capsules rapidly hydrated and attached to the mucin coating of the stomach. The experiment when repeated in dogs and human was much less effective due to a greater amount of soluble mucin in these animals as compared to rats. Stability problems of drugs or agents in the intestinal fluids can be overcome. Therapeutic effect of drugs insoluble in the intestinal fluid can be improved, especially in the case of drug acting locally. In this study, the evaluated mucoadhesive agents are used as adjuvant and as coating materials to develop oral mucoadhesive tablet20 for increasing residential time as well as absorption of drugs in gastrointestinal tract.
The influence of the putative bioadhesive polycarbophil on the gastric emptying of a pellet formulation20 was investigated by Khosla and Davis in 1987. The gastric emptying of pellets, labeled with a gamma – emitting radionuclide, was measured in human subjects using the technique of gamma scintigraphy. Similar rates of emptying for polycarbophil formulation and control formulation indicated that their admixture with polycarbophill did not retard the gastric emptying of pellets in fasted subjects. On the other hand, Russel and Bass reported that 50% of a 90g polycarbophil meal7 emptied within 4 canine gastric acid.
Ito R. et al8 developed magnetic granules containing ultrafine, brilliant blue FCP, and bioadhesive polymers (10 : 1 : 9 w/w), surmising a possible application for targeting therapy for esophageal cancer. When 5 mg of granules containing a mixture of HPC solution, about 90% of the granules were held in the region of the applied magnetic field when the granules were administered to rabbits with about 2 ml of 0.65% HPC solution via catheter and without anesthesia; nearly all of the granules were held in the region 2 hr after administration with magnetic guidance for the initial 2 min.
Aiache mixed morphine sulfate with a natural protein, Prosobel L 85 (50%) and a hydrophilic polymer, HPMC (0.5 – 1%), then wet granulated and prepare a sustained-release mucoadhesive dosage form22.
Decrosta et. al. used carbopol 934P as mucoadhesive substance to prepare captopril sustained-release tablets6. Captopril mixed with carbopol 934P and stearic acid (as lubricant), and tableted for sustaining the release of the drug for upto 16 hr more.
Matharu and Sanghavi aslo used carbopol 934P and poly acrylic acid cross – linked with 0.001% ethylene glycol to prepare mucoadhesive tablets7 for captopril.
EXPERIMENTAL RESEARCH WORK
Experimental analysis on mucoadhesiveness and mucoadhesion strength determination of different mucoadhesive agents (natural obtained from edible plants extracts and synthetic polymers) through comparison studies and selection of mucoadhesive agents for devising mucoadhesive tablet formulations was performed16.
MATERIAL AND METHODS
Materials
The mucoadhesive polymeric materials were used listed as follows in table 1 and table 2.
Methods
Studies regarding mucoadhesion strength determination were performed in different methods such as Falling ball method, Robinson’s method and Rabbit model method.
Table 1: Natural edible plants extracted mucoadhesive agents used
Table 2: Synthetic mucoadhesive agents / polymers used
|
Falling Ball Method: The mucoadhesive solution coated mustard seeds were passed through goat intestinal mucus solution (pH 5.5), U.S.P. simulated intestinal fluid U.S.P. (pH 6.0) and simulated gastric fluid U.S.P. (pH 1.2) at a specific distance i.e. 10 cm (figure 7). The time required to pass this specific distance for the coated seeds were noted. All experimentations were performed at room temperature. Before experimentation small variety of mustard seeds were coated with 0.75% w/v and 1.0% w/v mucoadhesive solutions in a small laboratory type coating pan. The coated seeds were swelled with water for 5 min. before the commencement of the experiment.

Robinson’s Method: A modified and suitably developed balance (figure 8) was used for estimating the tensile strength usually measure the force required to break the adhesive bond between a model membrane and the test mucoadhesive agents. The force required to separate bioadhesive sample from freshly excised goat intestinal tissue was determined using a modified tensiometer. A section of the goat intestinal tissue, having the mucus side exposed, was secured on a wetted rubber stopper placed in a beaker containing goat intestinal mucus solution or simulated gastric fluid U.S.P. or simulated intestinal fluid U.S.P. Another section of the same tissue was placed over a rubber stopper, again with the mucus side exposed. Then one drop of mucoadhesive solution was placed between the two mucosal tissues. The force used to detach the mucoadhesive solution adhered two sections of mucosal tissue was then recorded. The results of the study provided important information regarding the effects of charge density, hydrophobicity and experimental conditions such as pH, ionic strength, mucolytic agents, and applied pressure on bioadhesion. Experimentations were performed at room temperature. The water was poured into the container gradually upto just sufficient to detach two mucosal tissues. The volume or weight of water was measured and considered as adhesive strength of the used solution1.

Rabbit Model Method: Administration of Barium sulphate tablets based on mucoadhesive materials and comparison of adhesion and retention period of barium sulphate was observed. According to the reports of X-ray plates analysis the polymers were selected for development and devising mucoadhesive tablets containing model drugs.
RESULTS AND DISCUSSION
Graphical representation indicates the mucoadhesive strength of different mucoadhesive agents in Falling ball method (figure 9), Robinson’s method (figure 10) and Rabbit model method (figure 11).
The results of the studies provided important information regarding the effects of charge density, hydrophobicity and experimental conditions such as pH, ionic strength, mucolytic agents, and applied pressure on bioadhesion. Experimentations were performed at room temperature. The time of passing through solutions and weight of water or force to detach of mucoadhesion were estimated and recorded as per graphs in figure 9 and 10. The measured and recorded times and forces were considered as adhesive strength of the used solution and among the different mucoadhesive agents two plants extracts and two synthetic polymers were selected for mucoadhesive tablet formulations as binders and coating materials as per the greater strength.


This study is high lighting the use of two natural edible polymers / mucoadhesive agents obtained from jute leaf extract and vine spinach leaf extract, and two synthetic mucoadhesive polymers carbopol 934 and carbopol 940, for the formulation of gastrointestinal adhesive tablets16 after thorough studies on mucoadhesiveness of different natural and synthetic polymers / mucoadhesive agents (table 1 and 2). The 1.0% w/v solution of those mucoadhesive agents were used as binder for granulation of the tablets and the 3.0% w/v solutions were used as coating agents of the adhesive tablets of Barium sulphate. The barium sulphate oral mucoadhesive tablets16 were administered to the Rabbit. The adhesive tablets of Barium sulphate were adhered for prolong time to the mucosal absorptive membrane of the gastrointestinal tract of the Rabbit, that was observed by X-ray plate analysis of the Rabbit gastrointestinal tract after specific time of interval of tablet administration. The no. 1, 2, 4 and 6 indicate the times in hour for taking of X-ray plate and adhesion hours of barium sulphate in gastrointestinal tract of rabbit16.

The edible plant Vine spinach leaf extract and synthetic polymer Carbopol 940 were showing the retention period of barium sulphate in G.I.T upto 6 hours. The vine spinach leaf extract is having greater mucoadhesive strength than the Carbopol 940 (figure 11).
Table 3: Marketed oral and gastro-retentive controlled release novel drug delivery systems23,24
|
|
Marketed Controlled Release and Oral Mucoadhesive formulations23,24
Controlled release oromucosal gel containing drug Chlorhexidine hydrocortisone sodium succinate has been developed by Glaxo Smith Kline, available in market brand Corsodyl gel. Controlled release Corlan pellets containing drug Hydrocortisone sodium succinate is available in Oromucosal pallets dosage form. Controlled release Corlan pellets containing drug Hydrocortisone sodium succinate has been manufactured by Celltech Pvt. Limited. Quick release Sulbutex tablet is carrying combination of drugs Buprenorphine HCl and Naloxone which has been developed by Reckitt Benckiser. Controlled release tablet Prochlorperazine is available in brand name Buccastem. Buccastem has been manufractured by Reckitt Benckiser group. Tablet Straint SR is containing drug Testosterone has been prepared and marketed by Columbia Pvt. Limited. Quick release Zolpimist Spray carrying Zolpidem as main drug has been prepared by Nova Del Pharmaceuticals.
CONCLUSION
The special placements of drug formulations capable for providing prolong attachment for better bioavailability through long time absorption. Mucoadhesive agents/polymers have been utilized for retaining and localizing a drug product at a certain site for a certain time in the gastrointestinal tract or other body cavities. Controlled release mucoadhesive drug delivery systems specially gastro retentive drug delivery systems based on polymeric materials have been developed for the betterment in health care services by reducing the dosage and frequency of administration of drug, and improving the patient compliance despite the numerous advantages offered by these delivery systems. Mucoadhesive systems of drug delivery will be the potential alternative in near future in health care system.
ACKNOWLEDGEMENT
Very much grateful to Prof. (Dr.) A. K. Bandyopadhayay, Division of Pharmaceutics, Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India, and authority and animal ethical committee of Jadavpur University for providing the facilities to perform the work and approval of work. Thankful to University grant commission for providing the fellowship for completion of the work.
REFERENCES
- Nagai, T., & Konishi, R. (1987). Buccal/gingival drug delivery systems. Journal of Control Release, 6(1), 353–60. https://doi.org/10.1016/0168-3659(87)90088-5
- Mahajan, P., Kaur, A., Aggarwal, G., & Harikumar, S.L. (2013). Mucoadhesive Drug Delivery System- A Review. International Journalof Drug Development and Research, 5(1), 11-20.
- Ahuja, A., Khar, R.K., & Ali, J. (1997). Mucoadhesive drug delivery systems. Drug Development and Industrial. Pharmacy, 23, 489-515. https://doi.org/10.3109/03639049709148498
- Harris, D. & Robinson, J.R. (1992). Drug delivery via the mucous membranes of the oral cavity, Journal of pharmaceutical sciences, 81, 1-10. https://doi.org/10.1002/jps.2600810102 PMid:1619560
- Shakya, P., Satheesh, M.N.V. & Shakya, A.K. (2011). Kuldeep singh. Palatal mucosa as a route for systemic drug delivery: review. Journal of Controlled Release, 151, 2–9. https://doi.org/10.1016/j.jconrel.2010.11.003
PMid:21059376 - Decrosta, M.T., Jain, N.B. & Rudnic, E.M. (1987). S. Patent No.4666705.
- Matharu, R. S. & Sanghavi, N.M. (1992). Novel drug delivery system for captopril. Drug Development and Industrial Pharmacy, 18(14), 1567-1574. https://doi.org/10.3109/03639049209040859
- Ito, R., Mchida, Y., Sannan, T., & Nagai, T. (1990). Magnetic granules: a novel system for specific drug delivery to oesophageal mucosa in oral administration. International Journal of Pharmaceutics, 61,109-117. https://doi.org/10.1016/0378-5173(90)90049-A
- Rajesh, B. G., & Joseph R. R. (1988). Bioadhesion in Drug Delivery. Indian Journal of Pharmaceutical Sciecnces, 50(3), 145-152.
- Smart J. D. (1999). The role of water movement and polymer hydration in Mucoadhesion, in: Mathiowitz, E., Chickering, D.E. & Lehr, CM. (Eds.), Bioadhesive Drug Delivery Systems: Fundamentals, Novel Approaches and Development, Marcel Decker, New York.
- Jimenez-Castellanos, M. R., Zia, H. & Rhodes, C.T. (1993). Mucoadhesive Drug Delivery Systems, Dev. Ind. Pharm. 19 (1&2).143-194 (published online: 20 Oct. 2008, -Issue 1-2). https://doi.org/10.3109/03639049309038765
- Wachem, P.B.V., Beugeling, T., Feijen, J., Bantjes, A., Detmers, J.P. & Aken, W.G.V. (1985). Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials, 6(6), 403-408. https://doi.org/10.1016/0142-9612(85)90101-2
- Phanindra, B., Moorthy, B. K., & Muthukumaran, M. (2013). Recent advances in mucoadhesive/bioadhesive drug delivery system: A review. International journal of pharmaand bio sciences, 2(1), 68- 84.
- Dharmendra, S., Surendra, J.K., Sujata, M., Ashish, P. & Shweta, S. (2012). Mucoadhesive Drug Delivery System: A Review. International Journal of Pharmaceutical & Biological Archives, 3(6), 1287-1291.
- Tangri, P. et al. (2011). Oral mucoadhesive drug delivery system: A review. International Journal of Biopharmaceutics, 2(1), 36- 46.
- Sahana, B. (2002). Design and development of oral mucoadhesive drug delivery systems with some natural mucoadhesive agents. Jadavpur University.
- Huang, Y., Leobandung, W., Foss, A. & Peppas, N.A. (2000). Molecular aspects of mucoadhesion and bioadhesion: tethered structures and site specific surfaces. Journal of Controlled Release, 65, 63-71. https://doi.org/10.1016/S0168-3659(99)00233-3
- Longer, M.A., Ch’ng, H.S. & Robinson, J.R. (1985). Bioadhesive polymers as platforms for oral controlled drug delivery III: oral delivery of chlorothiazide using a bioadhesive polymer. Journal of Pharmaceutical Sciences, 74(4), 406-11. https://doi.org/10.1002/jps.2600740408
PMid:3999000 - Russell, J. & Bass, P. (1985). Canine gastric emptying of polycarbophil, an indigestible particulate substances. Gastroenterology, 89(2), 307-12. https://doi.org/10.1016/0016-5085(85)90330-0
- Khosla, R. & Davis, S.S. (1990). The effect of tablet size on the gastric emptying of non-disintegrating tablets. International Journal of Pharmaceutics, 62, R9-R11. https://doi.org/10.1016/0378-5173(90)90243-W
- E. Ballard, “Sustained and Controlled Release Drug Delivery System”, (J.R. Robinson ed.), Marcel Dekker, 1978.
- Aiache, J.M. (1992). International Patent Publication WO 92/02209.
- Tripathi, J., Thapa, P., Maharjan, R. & Jeong, S. H. (2019). Current State and Future Perspectives on Gastroretentive Drug Delivery Systems, Pharmaceutics, 11, 193. https://doi.org/10.3390/pharmaceutics11040193 PMid:31010054 PMCid:PMC6523542
- Garg S. and Sharma S. Gastroretentive Drug Delivery System, Business Briefing: Pharmatech. 2003, 160-166.

